Direct observation of strain-induced change in surface electronic structure on the Cu(001) surface
Daiichiro Sekiba, Kan Nakatsuji, Yoshiihde Yoshimoto and Fumio Komori
ref.) D. Sekiba, K. Nakatsuji, Y. Yoshimoto, and F. Komori: Direct Observation of Strain-Induced Change in Surface Electronic Structure. Phys. Rev. Lett. 94 (2005) 016808 (4).
Recent experimental studies have reported that chemical reactions on d-electron metal surfaces can be modified by the external strain [1]. Theoretical attempts to interpret these phenomena have been done on the basis of first-principles calculations of electronic structure, whereas there have been little experimental efforts to examine the electronic structure itself. We have adopted the Cu(001) surface as a model system, whose gnaturalh electronic structure is well-known, and demonstrated [2] the direct evidence of the strain-induced change of individual Cu3d states by angle-resolved photoemission spectroscopy.
We introduced a local strain field on the Cu(001) surface by nitrogen partial adsorption. At 0.3 mono-layer (ML) of the averaged nitrogen coverage, the surface is covered with a well-ordered array of the N-adsorbed domain as shown in Fig. 1. The clean Cu region imaged brightly is compressed because of the large lattice constant on the N-adsorbed domain. In a simple approximation, the Cu lattice deformation on average will be proportional to the nitrogen coverage.
Figure 2 shows the observed band dispersions around the M point on the clean, 0.1 ML, 0.2 M and 0.3 ML nitrogen-adsorbed surfaces. In these figures, we can see two kinds of electronic structure change: (1) the binding energy of the Tamm state decreases with increasing the nitrogen coverage, ie., the increase of the strain at the clean region, (2) the folding point of the Tamm state moves to a larger k-point with increasing the strain.
The experimental results were compared with the first-principles calculations with a symmetric slab model. A uniform compressive stress was given to the slabs laterally while interlayer relaxation due to the stress was allowed for the simulation of the strained Cu surface. The calculated energy shifts of the surface states excellently agree with the observation. Moreover, the shift of the folding point of the Tamm state is quantitatively explained by the sum of the Brillouin zone extension and the change of the work function due to the lattice constant reduction.
References
[1] for example, M. Gsell, et. al; Science 280 (1998) 717.
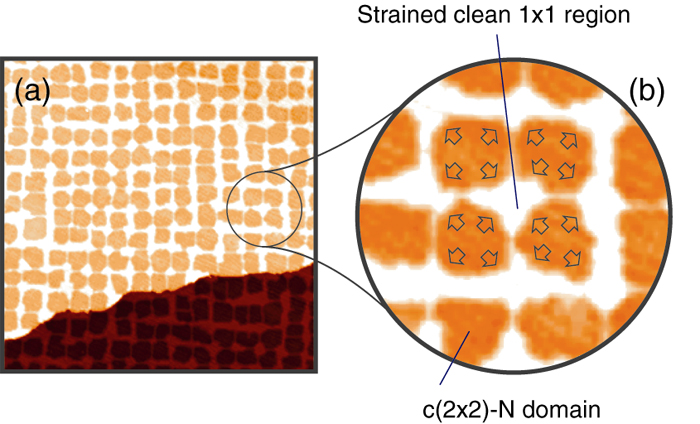
Figure 1: (a) STM image of the partially nitrogen-adsorbed Cu(001) surface. The Dark area is the N-adsorbed domain, and the bright part corresponds to the remaining clean region. The nitrogen coverage is 0.3 ML, and the image size is 100 nm x 100 nm. (b) Magnification of the STM image. The arrows schematically indicate the direction of atom replacement in the N-adsorbed domain. The clean surface is thus compressed.
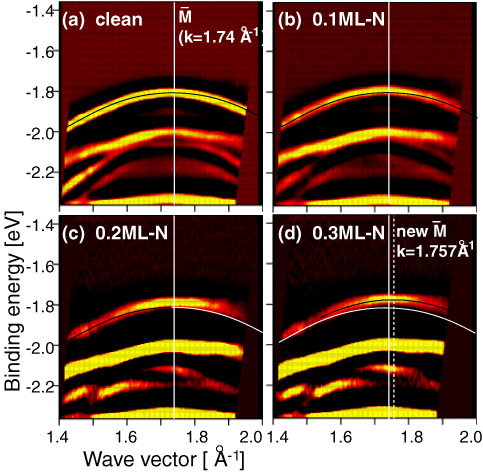
Figure 2: Band dispersions experimentally observed on the clean (a), 0.1 ML N-adsorbed (b), 0.2 ML (c) and 0.3 ML (d) surfaces. The solid curves in (a-c) and the white curves in (c,d) indicates the band dispersion of the Tamm state, a surface state, determined on the clean surface. The solid curve in (d) show the Tamm state band dispersion. The white line in each figure represents the folding point of the Tamm state on the clean surface, and the white dotted line in (d) shows the new folding point. Both the energy at M and the wave vector of the folding point shift with increasing the N-coverage.

